Advancing Knowledge on BRD, BVD, and Coccidia
Share
Advancing Knowledge on BRD, BVD, and Coccidia
Building on our previous discussion about the challenges of livestock health, this blog takes a deeper dive into the intricate world of key pathogens affecting cattle: Bovine Respiratory Disease (BRD), Bovine Viral Diarrhea (BVD), and coccidia. These are not just health issues; they represent critical threats to herd well-being and farm profitability. By exploring the nature of these diseases, their far-reaching impacts, and the latest advancements in veterinary medicine, we aim to spark curiosity and empower cattle farmers. Understanding the complexities behind these conditions isn’t just beneficial; it’s transformative. Through knowledge, we unlock the tools for proactive herd management, ensuring healthier Highland, minimizing losses, and fostering resilience in the face of inevitable challenges. Join us on this journey to elevate your understanding and approach to cattle health.
Understanding BRD Pathogens
Bovine Respiratory Disease (BRD) is a multifaceted condition caused by a combination of bacterial and viral pathogens that work in tandem to comprise the respiratory health of Highland. The disease is often exacerbated by environmental stressors such as overcrowding, inadequate ventilation, sudden weather fluctuations, or transportation stress—factors that weaken the immune system and create ideal conditions for pathogens to thrive. As one of the leading causes of morbidity and mortality in cattle, BRD presents significant challenges for a fold health and farm profitability. These pathogens specifically target the respiratory system, leading to inflammation, fluid buildup, and impaired oxygen exchange. If not addressed promptly, BRD can result in long-term health issues, decreased growth rates, and substantial economic losses due to reduced productivity and increased veterinary costs. Understanding the complex interplay of pathogens and stressors in BRD is crucial for effective prevention and management strategies.
Key Bacterial Pathogens
- Mannheimia haemolytica: This bacterium is infamous for causing severe fibrinous pneumonia, particularly in young calves. It is highly opportunistic, exploiting weakened immune systems during periods of stress, such as transport or mixing cattle from various sources. Once introduced, it rapidly colonizes the respiratory tract, releasing potent toxins that damage lung tissue. This leads to extensive inflammation and fluid buildup, impairing oxygen exchange. The disease progression can be swift, often resulting in noticeable respiratory distress within Hours. Early detection and intervention are crucial, as delayed treatment significantly increases mortality risks.
- Pasteurella multocida: This pathogen is a versatile bacterium often linked to chronic respiratory conditions but can also provoke acute disease under specific circumstances. It becomes particularly problematic when cattle are subjected to stressors such as transportation, extreme weather changes, or overcrowding. These stressors weaken the immune system, allowing P. multocida to proliferate and invade lung tissues. The result is inflammation, mucus accumulation, and in severe cases, bacterial pneumonia. Unlike more aggressive pathogens like Mannheimia haemolytica, P. multocida typically causes slower-onset symptoms but can rapidly escalate when paired with viral infections, such as BVD or IBR, leading to compounded respiratory challenges.
- Histophilus somni: This bacterium is a versatile and opportunistic pathogen that impacts multiple systems in cattle. While it often starts as a respiratory infection, it has the ability to enter the bloodstream, spreading throughout the body and causing severe complications. Once in the circulatory system, it can localize in joints, leading to painful swelling, lameness, and reduced mobility. In some cases, it invades the central nervous system, resulting in brain inflammation or thromboembolic meningoencephalitis (TEME). This condition manifests as uncoordinated movements, head tilting, or even paralysis. The rapid progression of systemic infections caused by H. somni can lead to sudden death, particularly if not diagnosed and treated early. Effective management requires prompt antibiotic intervention and addressing any predisposing factors, such as stress or co-infections, that compromise immune defenses.
- Mycoplasma bovis: Unique among bacterial pathogens, Mycoplasma bovis lacks a cell wall, a feature that makes it inherently resistant to antibiotics like beta-lactams, which target cell wall synthesis. This pathogen is known for causing chronic pneumonia, which manifests as persistent coughing, nasal discharge, and labored breathing that often fail to respond to standard treatments. In addition to respiratory issues, it is a leading cause of arthritis in cattle, resulting in swollen joints, pain, and lameness that significantly impair mobility and productivity. Another hallmark condition associated with M. bovis is mastitis, particularly in dairy cattle, where it leads to decreased milk production, poor milk quality, and recurrent infections that are challenging to eradicate. The chronic and multifaceted nature of Mycoplasma bovis infections underscores the importance of early diagnosis, targeted antibiotic use, and robust biosecurity measures to prevent its spread within herds.
Key Viral Pathogens
- Bovine herpesvirus-1 (IBR): This virus is a highly contagious pathogen that targets the upper respiratory tract, leading to inflammation and damage to the mucosal lining. The compromised respiratory tract loses its natural defenses, such as mucus production and ciliary action, which are essential for clearing bacteria and other pathogens. As a result, secondary bacterial infections, including pneumonia caused by Mannheimia haemolytica or Pasteurella multocida, frequently follow an IBR outbreak. Infected cattle often exhibit nasal discharge, fever, coughing, and conjunctivitis, with symptoms worsening if secondary infections develop. Early detection and supportive care are critical in managing IBR outbreaks, along with vaccination to reduce the severity and spread of the disease.
- Bovine Viral Diarrhea Virus (BVD): A critical player in BRD complexes, BVD significantly impacts cattle health by targeting and suppressing the immune system. This immunosuppression increases the animal’s vulnerability to secondary bacterial pathogens, such as Mannheimia haemolytica and Pasteurella multocida. BVD interferes with normal immune functions by reducing white blood cell counts and impairing their ability to respond to infections. This suppression not only predisposes cattle to respiratory illnesses but also affects their overall ability to fight off diseases, leading to more severe outbreaks within herds. In addition, BVD infections can exacerbate pre-existing conditions, complicate recovery, and lead to higher mortality rates if not managed effectively. Vaccination and robust herd management are essential to minimize the impact of this pervasive virus.
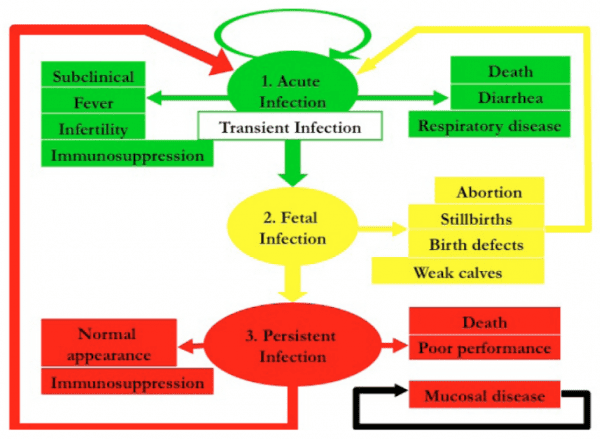
A Closer Look at BVD
BVD is a highly contagious viral disease with profound implications for cattle health and herd productivity. It spreads easily through direct contact with infected animals or contaminated materials such as feed, water, and equipment. This disease affects cattle of all ages and can manifest in different forms, including acute, chronic, and persistent infections, each presenting unique challenges. Understanding its diverse manifestations—ranging from immune suppression to severe reproductive impacts—and its pathways of transmission is essential for implementing effective control measures and minimizing its impact on herd health and economic sustainability.
Acute BVD:
- Symptoms include a sudden onset of high fever, often exceeding 105°F, accompanied by sweating, shivering, and visible discomfort. Watery or bloody diarrhea is a hallmark sign, with the presence of blood indicating significant intestinal damage. Painful ulcers in the mouth or nose can lead to drooling and difficulty eating, further exacerbating weight loss. Rapid weight loss is often noticeable within days, as the animal’s energy reserves are depleted due to reduced feed intake and severe dehydration. Lethargy becomes pronounced, with affected animals isolating themselves, lying down more frequently, and exhibiting little to no response to external stimuli. In severe cases, systemic collapse and death can occur within a short time frame without intervention.
- Reproductive impacts are notable, with affected cows often experiencing abortions, stillbirths, or congenital defects in calves.
Chronic BVD:
- Chronic cases of BVD often manifest as persistent diarrhea that may range from mild to severe, leading to consistent loss of fluids and nutrients. This results in significant weight loss, as the animal’s digestive system fails to efficiently absorb the necessary energy and nutrients from feed. Recurrent infections are a hallmark of chronic BVD, as the virus suppresses the immune system, leaving affected animals vulnerable to secondary bacterial or viral diseases. These animals are often referred to as "poor doers," as they struggle to maintain body condition despite adequate nutrition and care. Unlike acute BVD, which has more immediate and dramatic symptoms, chronic BVD can go unnoticed initially, with the gradual decline in health becoming evident only over time. These differences highlight the need for vigilant herd monitoring and proactive veterinary intervention to prevent long-term herd productivity losses.
BVD-PI (Persistent Infection):
- Occurs when a fetus is infected in utero during the 2nd trimester of gestation, a critical window for immune system development. During this period, the calf’s immune system is still learning to distinguish between "self" and "non-self." As a result, the virus integrates into the calf’s immune blueprint, leading to the immune system recognizing the virus as part of its own body, not an invader. This mistaken identity results in lifelong viral shedding, where the calf continuously releases the virus into the environment through saliva, nasal secretions, urine, feces, and even milk. This shedding is EXPENDENTAL. Not just double but 1000 times more (as my vet explained to me. Leaving others in your pasture Highly exposed! Unlike acute BVD, which manifests with clear and often severe symptoms, BVD-PI animals may appear outwardly healthy but are a constant source of infection within the herd. Their presence poses significant risks for herd health, making identification and management crucial to controlling the spread of BVD.
- PI animals are asymptomatic carriers, meaning they do not display visible signs of illness despite harboring and shedding the virus continuously. This characteristic makes them the primary vectors for BVD transmission within herds. These animals release the virus through saliva, nasal secretions, urine, feces, and even milk, contaminating shared resources such as feed and water. This constant viral shedding poses a persistent risk of infection to other cattle, particularly young calves and pregnant cows. Detecting PI animals requires precise diagnostic testing, such as antigen capture ELISA or PCR tests, as their outwardly healthy appearance can be misleading. Removing these animals from the herd is not only critical for disease control but also for breaking the cycle of transmission, ultimately safeguarding the health and productivity of the entire herd. Known PI infected animals require special dispersal by certified buyers.
Coccidia: A Silent Threat
Coccidia are protozoan parasites from the genus Eimeria. Unlike bacteria and viruses, protozoans are single-celled eukaryotic organisms, meaning they have a more complex cell structure, including a nucleus and specialized organelles. This complexity allows them to adapt and survive in challenging environments, such as the intestinal lining of cattle, where they silently invade and cause significant damage. Unlike bacteria, which are prokaryotic and lack a nucleus, or viruses, which are even simpler and require a host cell to replicate, protozoans like coccidia have intricate life cycles that include multiple stages, some of which are highly resistant to environmental conditions. Their ability to disrupt nutrient absorption and overall health, especially in young cattle, makes them a persistent and challenging threat to manage.
Transmission:
- Spread primarily through the fecal-oral route, with oocysts—the hardy, infectious stage of the parasite—shed in feces contaminating feed, water, bedding, and other shared surfaces. These oocysts are highly resistant to environmental conditions, allowing them to survive for extended periods in soil, water, or fecal material. This resilience means that even minor lapses in hygiene can lead to significant outbreaks, particularly in overcrowded or unsanitary environments. Additionally, oocysts can adhere to equipment, boots, or the fur of animals, further facilitating their spread across different areas of a farm. Overcrowding, damp conditions, and poor sanitation amplify the risk, creating ideal circumstances for oocysts to thrive and infect other animals.
- Overcrowding, damp conditions, and poor sanitation significantly increase the risk of outbreaks by creating an environment where infectious agents like oocysts can thrive and spread rapidly. In overcrowded spaces, cattle have limited personal space, leading to higher contact rates and increased exposure to contaminated surfaces or feed. Damp conditions further exacerbate the problem by providing moisture that helps sustain the viability of oocysts, while poor sanitation allows waste to accumulate, turning shared living areas into reservoirs for infection. Together, these factors create a perfect storm for outbreaks, emphasizing the critical need for proactive management practices to reduce these risks.
Symptoms:
- Bloody or mucus-filled diarrhea is one of the most distinctive signs of coccidia infection, often presenting as a yellowish, watery consistency with a foul metallic odor. This unusual smell is a result of the severe damage to the intestinal lining and the associated metabolic changes in the gut. The diarrhea can contain blood or mucus, indicating significant intestinal inflammation and sloughing of tissue. Dehydration quickly follows due to the rapid loss of fluids, leaving the animal with sunken eyes, dry mucous membranes, and reduced skin elasticity. Weight loss becomes apparent as the infected cattle struggle to absorb nutrients, further compounded by reduced feed intake. These symptoms are hallmark indicators of a severe coccidia infection and require immediate attention to prevent further deterioration.
- Severely affected animals may exhibit profound lethargy, often isolating themselves from the herd and lying down for extended periods. Stunted growth becomes evident as the animals fail to gain weight or maintain proper body condition despite adequate feed availability. Decreased appetite exacerbates these issues, with affected cattle showing little interest in grazing or supplemental feed, further compounding their energy deficits. These symptoms directly contribute to significant losses, as poor performance not only reduces productivity but also increases veterinary costs and prolongs recovery times, ultimately impacting overall viability.
Treatment and Prevention:
- Treatment: Anticoccidial drugs, such as amprolium (commonly known as Corid) or sulfa-based medications like sulfadimethoxine (Albon), target specific stages of the parasite’s life cycle, inhibiting its ability to replicate and spread. These medications are particularly effective when administered early in the course of the infection. Severe cases, where dehydration and nutrient loss are significant, may necessitate supportive care such as intravenous or oral fluid therapy to restore hydration and electrolyte balance. This comprehensive approach not only alleviates the symptoms but also minimizes long-term damage to the intestinal lining.
- Maintain clean, dry living conditions by providing well-drained bedding and ensuring adequate ventilation to reduce moisture levels. Implement regular manure removal protocols to prevent the buildup of waste, which can serve as a breeding ground for pathogens like coccidia. Frequent cleaning of feeding and watering areas is also crucial to minimize contamination and ensure a healthier environment for the herd. Additionally, employing rotating grazing practices and maintaining appropriate stocking densities can further reduce the risk of disease spread and promote overall herd health.
- Quarantine infected animals immediately to limit environmental contamination and prevent further spread of the infection. Isolated animals should be housed in designated areas away from the main herd, with separate feeding and watering systems to reduce cross-contamination. Regular monitoring of quarantined cattle is essential to assess recovery and ensure they are not shedding pathogens before reintroduction. This practice not only protects healthy animals but also provides an opportunity to evaluate and improve biosecurity measures across the farm.
- Optimize nutrition to strengthen immune defenses, as a well-balanced diet rich in essential vitamins and minerals plays a vital role in maintaining overall health and bolstering the immune system. Providing high-quality forage, adequate protein, and supplements like zinc, selenium, and vitamin E can enhance the body’s ability to ward off infections. Healthier animals with stronger immune systems are less likely to succumb to severe infections, recover faster when exposed to pathogens, and contribute to a more resilient and productive herd.
Treatment Options
1. Antibiotics for BRD
- Target secondary bacterial infections using options like Florfenicol (Nuflor), a broad-spectrum antibiotic effective against respiratory pathogens, Tilmicosin (Micotil), known for its extended activity against key BRD pathogens, or Oxytetracycline (LA-300), a versatile antibiotic that addresses a wide range of bacterial infections. These medications work by inhibiting bacterial protein synthesis or cell function, effectively halting the infection's progression. Early and accurate administration under veterinary guidance ensures optimal results while minimizing resistance risks.
2. Antiviral Strategies for BVD
- Antiviral medications for BVD remain limited, as no specific antiviral treatments have been widely developed or approved for cattle. Instead, management focuses on supportive care to bolster the animal’s natural immune response. This includes ensuring proper hydration through oral or intravenous fluids, providing high-quality and nutrient-rich feed to support energy needs, and minimizing stressors such as overcrowding or environmental changes. Supportive care also involves isolating infected animals to reduce the spread of the virus and prevent co-infections, which could worsen the animal’s condition. This holistic approach helps improve recovery rates while mitigating the broader impact of the disease on the herd.
- Vaccines, particularly modified-live versions, are critical tools for disease prevention, offering one of the most effective methods to protect cattle from devastating illnesses like BVD. These vaccines work by stimulating the immune system to recognize and respond to pathogens before they can cause harm. However, they must be used judiciously to avoid adverse effects, such as reactions in pregnant cows or interactions with other treatments. Prevention through vaccination is not just a safeguard for individual animals but a cornerstone of herd health. By reducing the prevalence of disease-causing agents within the population, vaccination creates a healthier and more resilient herd, minimizing losses and ensuring long-term sustainability.
3. Supportive Care
- Providing a low-stress environment and isolating sick animals are foundational for recovery, as stress significantly impacts an animal's immune response and overall healing capacity. Stress reduction can be achieved by ensuring a calm, quiet space with minimal disturbances and maintaining comfortable temperatures and ventilation. Isolating sick animals not only prevents the spread of illness to healthy herd members but also allows focused care, such as specialized feeding and hydration schedules. This dual approach fosters a conducive environment for quicker recovery and improved health outcomes.
- Anti-inflammatory drugs (NSAIDs) help reduce fever and inflammation, improving comfort and outcomes. In addition to NSAIDs, hydration therapy plays a crucial role in the recovery process. Providing oral electrolyte solutions helps restore fluid balance and prevent dehydration caused by severe diarrhea or illness. Intravenous (IV) fluids may be necessary for more severely affected animals to ensure rapid rehydration and stabilization. It is important to note that electrolytes should not be administered within four hours of milk feeding to avoid interference with digestion and nutrient absorption. These combined measures not only alleviate immediate symptoms but also support the overall recovery process by addressing underlying physiological needs.
- Dehydration can lead to life-threatening conditions, primarily through the thickening of blood and the loss of its ability to carry oxygen efficiently. As the animal loses fluids, blood viscosity increases, which places additional strain on the heart and circulatory system. Reduced oxygen delivery to vital organs such as the brain, liver, and kidneys can result in systemic organ failure. Recognizing and addressing dehydration promptly with appropriate therapies is critical to preventing these severe outcomes and ensuring the animal’s survival.
Innovations in Veterinary Medicine
1. Mechanisms of Action
- Modern antibiotics, such as fluoroquinolones (e.g., enrofloxacin marketed as Baytril), target DNA replication enzymes like DNA gyrase and topoisomerase IV. By interfering with these critical enzymes, these antibiotics prevent bacterial cells from replicating their DNA, halting their growth and survival. This mechanism of action is highly effective against a broad spectrum of bacterial pathogens, including those resistant to other antibiotic classes. The precision of fluoroquinolones ensures targeted treatment, reducing the pathogen load while preserving beneficial microbiota.
2. Nanoparticle-Based Delivery Systems
- Advances in nanotechnology are revolutionizing drug delivery by enhancing targeting precision, ensuring that therapeutic agents achieve higher concentrations directly at infection sites while minimizing systemic side effects. These nanoparticle-based systems encapsulate medications, protecting them from premature degradation and enabling controlled, sustained release at the site of infection. This targeted approach not only increases the efficacy of the treatment but also reduces the risk of adverse effects on non-target tissues, ultimately leading to improved outcomes and reduced treatment costs in veterinary medicine.
3. Antibiotic Resistance
- Due to the ongoing mutations in bacterial vectors, many over-the-counter drugs have become increasingly ineffective against certain pathogens. These mutations alter the structure of bacterial enzymes or cell walls, rendering traditional antibiotics obsolete in many cases. This shift highlights the importance of veterinary oversight and the use of updated, prescription-based treatments to ensure effective disease management.
4. Changes in Prescription Medicine
- Maintaining a strong working relationship with a large animal veterinarian is essential for effective livestock management. A skilled vet provides expertise in diagnosing illnesses, administering treatments, and guiding preventative measures such as vaccination protocols. This partnership ensures timely interventions for health issues and access to prescription medications tailored to the specific needs of the herd. A trusted vet can help recommend advanced solutions, such as precision-targeted vaccines and long-acting medications, that enhance efficacy and streamline disease management. Investing in this collaboration not only safeguards herd health but also supports long-term farm productivity and sustainability.
Conclusion
The pathogens behind BRD, BVD, and coccidia present ongoing challenges for cattle farmers. However, Knowledge remains the most powerful tool for prevention and control. By understanding these diseases, their symptoms, and the latest treatment advancements, producers can build resilient herds and minimize losses. While this is a lot to take in, it underscores the importance of taking action.
Trust your instincts—if you feel something is off with one of your Highland cattle, even if you can’t explain it beyond them looking “wrong” or behaving differently, Act on that intuition. You know your animals better than anyone else.
Knowledge empowers you to make informed decisions, seek timely veterinary care, and implement proactive measures that safeguard not just individual animals but the health and future of your entire fold.
Education empowers, turning challenges into opportunities for growth and success.
We want to thank you for taking the time to learn and grow with us, becoming an integral part of the Highland community. Your dedication to your herd and your willingness to embrace new knowledge make a profound difference. Remember, you are not alone in this journey—you are part of the Triple M family, and together, we can achieve greatness in cattle care and management.
We ARE Stronger Together!
______________________________________________________________________
References
Cornell University College of Veterinary Medicine. (n.d.). Best management practices for biosecurity in cattle herds. Cornell University. Retrieved from https://www.vet.cornell.edu
MSD Veterinary Manual. (n.d.). Preventative health care and husbandry of beef cattle. MSD Veterinary Manual. Retrieved from https://www.msdvetmanual.com
Oklahoma State University Extension. (n.d.). Livestock mortality management. Oklahoma State University. Retrieved from https://extension.okstate.edu
United States Department of Agriculture. (2015). Death loss in U.S. cattle and calves due to nonpredator causes, 2015. National Animal Health Monitoring System. Retrieved from https://www.aphis.usda.gov
W.E. Jameson & Son. (n.d.). Coccidiosis risk factors. W.E. Jameson & Son. Retrieved from